Which of the Following Bonds Is the Least Polar
B Dipole moments result from the unequal distribution of electrons in a molecule. Two very e- atoms are covalently bound c.

Which Bond Is More Polar Youtube
Select the answer choice that correct identifies if the bonds formed would be nonpolar covalent polar covalent or ionic.
. Advertisement Advertisement New questions in Chemistry. Which of the following bonds is least polar. Which of the following bonds is least polar.
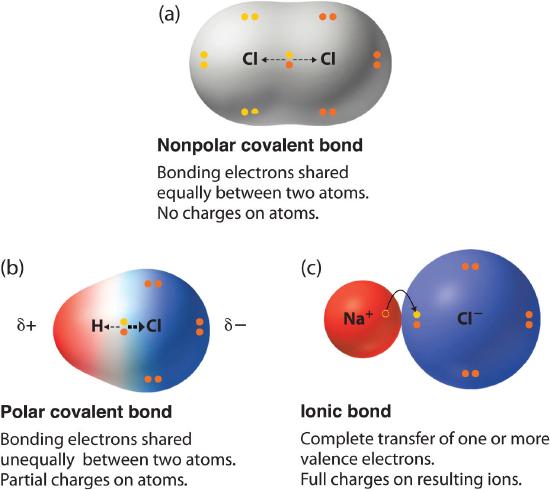
The electrons in a polar bond are found nearer to the more electronegative element. A Ionic bonding results from the transfer of electrons from one atom to another. Which of the following statements is incorrect.
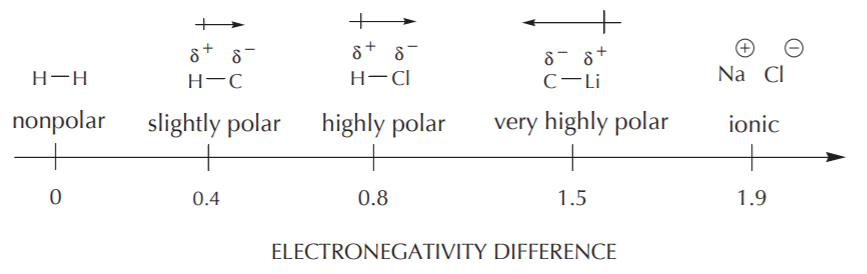
Which of the following single bonds is least polar. The electronegativity difference in oxygen and flourine is 05 and it is the least polar when you compare to all others. H-C is the correct answer as the electronegativity of the two atoms is closest but not the same making it the least polar bond.
Try to use a. Which of the following statements best describes a relatively polar bond. Two weakly e- atoms undergo ionic bonding b.
A CO b HC c PCl d NaCl e They are all nonpolar. Which of the following statements is incorrect. Na is about 09 and Cl is about 35 and the difference is about 26.
Thus the N-H bond is must less polar than the NaCl bond. E They are all nonpolar. Which of the following bonds would be the least polar yet still be considered polar covalent.
D A molecule with very polar bonds can be. Mg-O C-O O-O Si-O N-O. A very e- atom and a weakly e- atom are covalently bound d.
O-H 2 See answers Advertisement Advertisement gracesustiana gracesustiana Answer. C The electrons in a polar bond are found nearer to the more electronegative element. None of the above.
N is about 30 and H is 21 so the different is about 09. Two very e- atoms undergo ionic bonding e. See the answer See the answer done loading.
Find an answer to your question Place the following covalent bonds in order from least to most polar. Ionic bonding results from the transfer of electrons from one atom to another. The smallest difference will be the most covalent bonds least polar.
Dipole moments result from the unequal distribution of electrons in a molecule. But we know of course that we call NaCl an ionic bond and NH3 bonds covalent.

Ch 11 Quiz Bonding Electronegativity And Lewis Structures A
1 9 Electronegativity And Bond Polarity Review Chemistry Libretexts
Comments
Post a Comment